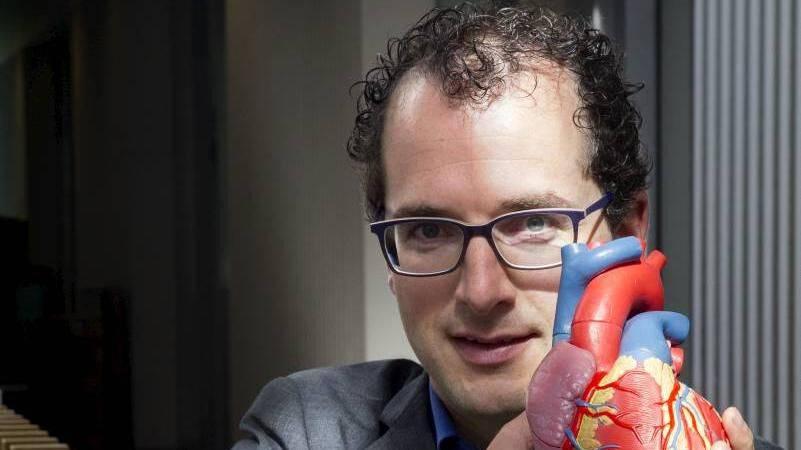
Al enkele jaren leven 18 kinderen verspreid over Europa, Azië en de VS met een geïmplanteerde, regeneratieve hartklep van het Eindhovense bedrijf Xeltis. Xeltis ontstond dertien jaar geleden als spin-off van de Universiteit Eindhoven en is daar nog altijd gevestigd, bij Twice | hub Catalyst. In een interview met het vaktijdschrift LABinsights, vertelt Martijn Cox, chief technology officer bij Xeltis, over dit sterk staaltje supramoleculaire chemie gecombineerd met regeneratieve geneeskunde.
Bibliotheek aan materialen
“De supramoleculaire chemie stelt ons in staat om een bibliotheek aan materialen te ontwikkelen, variërend van heel stijve tot heel rekbare materialen, van heel snel tot heel langzaam afbreekbare stoffen. Het is een veel bredere range aan stoffen dan je met gewone chemie zou kunnen bereiken”, vertelt Martijn Cox, chief technology officer en oprichter van het bedrijf in 2007. “Het verschil tussen supramoleculaire en gewone polymeren is dat we bij supramoleculaire materialen gebruik maken van relatief korte polymeren die we als een soort legoblokjes aan elkaar kunnen vastmaken met waterstofbruggen. Dat is een principe dat in de natuur bijvoorbeeld ook wordt gebruikt bij DNA. Waterstofbruggen zijn op zich niet zo sterk, maar als je een eenheid gebruikt met meerdere waterstofbruggen naast elkaar, dan zorgt dat voor een sterke en reversibele verbinding. Hierdoor zijn deze materialen veel sterker dan je zou verwachten op basis van hun molecuulgewicht”, legt Cox uit.
Weefselherstel
Xeltis gebruikt dit soort materialen om hartkleppen en stukjes bloedvat te maken die bij mensen geïmplanteerd kunnen worden, vertelt Cox. “We zien dat onze implantaten vanaf dag één functioneren zoals het lichaamsdeel dat ze vervangen. Maar tegelijkertijd zijn ze ook poreus, al zie je dat niet met het blote oog. Daardoor lekken de geïmplanteerde bloedvaten de eerste paar minuten na implanteren. Maar al snel daarna stolt het bloed in het materiaal en nestelen zich daar cellen in. Het gaat dan bijvoorbeeld om cellen uit omliggend weefsel en in het bloed circulerende cellen zoals macrofagen en fibroblasten. Eigenlijk gebeurt er hetzelfde wat ook gebeurt bij weefselherstel bij een wond.”
Na verloop van tijd is de kunststof helemaal gevuld en bedekt met lichaamseigen weefsel. “Na ongeveer een half jaar verliest de kunststof aan sterkte door oxidatie van bepaalde bindingen, waardoor het eigenlijk in brokjes uiteenvalt. Doordat het dan helemaal ingepakt zit in het nieuwe weefsel, kan het nog jaren duren voor het helemaal verdwenen is.”
Functioneel als natuurlijke hartklep
Wat overblijft is dan een nieuwe hartklep of bloedvat van lichaamseigen cellen. “Je zult altijd wel kunnen zien dat het niet hetzelfde is als een natuurlijke hartklep. De geïmplanteerde hartklep is bijvoorbeeld iets dikker en stijver, maar er zijn wel heel veel overeenkomsten en in essentie is er geen verschil in functioneren”, zegt Cox. “Net als een natuurlijke hartklep is er bijvoorbeeld een laagje cellen dat ervoor zorgt dat er geen stolsels ontstaan, en zijn er haarvaatjes door het materiaal en netwerken van eiwitten zoals collageen en elastine.”
Succes bij kinderen
Xeltis heeft zich in eerste instantie gericht op de ontwikkeling van hartkleppen voor kinderen. In een eerste klinische trial hebben twaalf kinderen uit Europa en Azië zo’n hartklep van Xeltis gekregen. Bij de eerste resultaten bleek dat er nog sprake was van een lichte complicatie, hoewel de kinderen daar weinig van merkten. Nadat de hartkleppen iets zijn aangepast, is vorig jaar een nieuwe trial gestart bij zes kinderen uit de VS. Tot nu toe is daar geen enkele complicatie opgetreden, zo maakten de onderzoekers afgelopen februari bekend. “We hopen dat het lichaamseigen weefsel uiteindelijk ook met de kinderen meegroeit, maar daar is nu nog geen bewijs voor. Maar zelfs als dat niet zo is, dan is onze hartklep al een verbetering ten opzichte van de huidige hartkleppen, die om de paar jaar vervangen moeten worden vanwege slijtage”, zegt Cox.
Hoge en lage druk
Ondertussen zijn medewerkers van Xeltis druk bezig met de ontwikkeling van andere varianten. Terwijl het bij de eerste trial ging om een hartklep aan de lagedrukzijde van het hart, werkt het bedrijf nu ook aan een hartklep voor de hogedrukzijde. Daarvoor worden meer eisen gesteld aan het materiaal. Bovendien wordt er gewerkt aan de ontwikkeling van een bloedvat voor een bypassoperatie, waardoor het niet meer nodig is om een bloedvat te gebruiken uit het been van de patiënt. De verwachting is dat eind 2020 de eerste klinische trials daarmee van start gaan.
Dit artikel verscheen op 26 juni 2020 in LABinsights.